Difference between revisions of "Description of the electropolishing process"
(New page: Back to Electropolishing in metal industry '''Electropolishing''' Electropolishing is a commonly used electrochemical method for smoothing, polishing, deburring and cleaning variou...) |
m (Changed protection level for "Description of the electropolishing process" ([edit=sysop] (indefinite) [move=sysop] (indefinite))) |
||
| (2 intermediate revisions by the same user not shown) | |||
| Line 19: | Line 19: | ||
''Source: BAT Surface Treatment of Metals and Plastic, Aug. 2006.'' | ''Source: BAT Surface Treatment of Metals and Plastic, Aug. 2006.'' | ||
| + | |||
| + | |||
| + | [[Image: Electropolishing with electric discharge.jpg]] | ||
| + | |||
| + | Source: http://www.anopol.co.uk/ | ||
| + | |||
| + | |||
| + | Technologies with exploited electropolishing features Aerospace (e.g. Crack detection in castings), Automotive (e.g. External wire-mesh car trim), Chemical (e.g. Polymeriser vessels), Semiconductor (e.g. Pipework and fittings for gases), Food and beverage (e.g. Hot water tanks), Hospital (e.g. Sterile furniture), Surgical (e.g. Instruments and implants), Marine (e.g. Boat handrails and fittings), Textile (e.g. Dye vats), Paper and Pulp (e.g. Screen cylinders), Nuclear (e.g. Plasma producing Torus), High Vacuum (e.g. Vacuum chambers), Pharmaceutical (e.g. Process tanks, pipes and valves), Architectural (e.g. Lampposts and sculptures), Leisure (e.g. Swimming pool ladders). | ||
| + | |||
| + | Source: http://www.anopol.co.uk/ | ||
| + | |||
Back to [[Electropolishing in metal industry]] | Back to [[Electropolishing in metal industry]] | ||
Latest revision as of 17:08, 13 February 2013
Back to Electropolishing in metal industry
Electropolishing
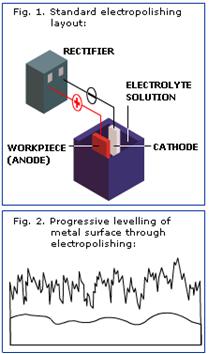
Electropolishing is a commonly used electrochemical method for smoothing, polishing, deburring and cleaning various metals, generally steel, stainless steel, copper and its alloys and aluminium and its aluminium alloys. It is widely used in food equipment, surgical equipment and implants, the pharmaceutical, paper, pulp and food-industries, as well as in automotive and architectural applications. Electropolishing removes a fine surface layer electrolytically, and is often used in cases where very smooth and bright finishes are needed. In electropolishing, the workpiece (anode) is immersed in electrolyte and electric current (usually DC) is connected between the workpiece and the cathode. The workpiece becomes polarised and metal ions start to diffuse to the cathode, and metal is removed from the anode. The reaction can be controlled by adjusting bath and process parameters and by choosing the metal or alloy being electropolished.
In these electropolishing processes, different electrolytes are used. Electrolytes are usually mixtures of various acids (sulphuric acid, chrome acid, citric acid, and/or phosphoric acid) and sometimes organic compounds (such as glycerine or diethyleneglycolmonobutylether) are added.
During electropolishing of stainless steels, hydrogen is formed, which mixes with oxygen at the solution surface. If ignited by a spark, this can produce an explosion. It is therefore advisable to extract gases forming on top of the solution. This is vital when processing the internal surfaces of enclosed vessels, otherwise a serious, possibly fatal, injury may occur. Repeated noise of this type may have an impact beyond the perimeter of the installation (as well as being an occupational health issue).
Source: BAT Surface Treatment of Metals and Plastic, Aug. 2006.
Electropolishing with electric discharge (also known as plasma – electrolytic polishing)Plasma-electrolytic polishing is an alternative method for some applications. The process differs from conventional electropolishing mostly because of its electrolytes and process parameters used. Instead of mixed acids, these electrolytes are different salt solutions and far more friendly for employers and for environment. In this process, the used electric potential between anode and cathode is in the range of 200 – 400 V DC depending on the solution and temperature (40-95°C) used.
The same process can be used also for plasma-electrolytic oxidation in order to get hard oxide-ceramic coatings.
Source: BAT Surface Treatment of Metals and Plastic, Aug. 2006.
Source: http://www.anopol.co.uk/
Technologies with exploited electropolishing features Aerospace (e.g. Crack detection in castings), Automotive (e.g. External wire-mesh car trim), Chemical (e.g. Polymeriser vessels), Semiconductor (e.g. Pipework and fittings for gases), Food and beverage (e.g. Hot water tanks), Hospital (e.g. Sterile furniture), Surgical (e.g. Instruments and implants), Marine (e.g. Boat handrails and fittings), Textile (e.g. Dye vats), Paper and Pulp (e.g. Screen cylinders), Nuclear (e.g. Plasma producing Torus), High Vacuum (e.g. Vacuum chambers), Pharmaceutical (e.g. Process tanks, pipes and valves), Architectural (e.g. Lampposts and sculptures), Leisure (e.g. Swimming pool ladders).
Source: http://www.anopol.co.uk/