Distillation in chemical industry
Back to EFFICIENCY FINDER FOR CHEMICAL INDUSTRY
1. OBJECTIVE
Distillation is the separation of the components of a liquid mixture by partial vaporisation of the mixture and separate recovery of the vapour and residue. The more volatile components of the original mixture are obtained at higher concentration in the vapour, the less volatile in higher concentration in the liquid/solid residue
2. FIELD OF APPLICATION
Distillation can be used for the separation of components in the products after the chemical reactions, such as halogenation and reduction of aromatic nitro compounds.
3. DESCRIPTION OF TECHNIQUES, METHODS AND EQUIPMENT
- Principal methods of distillation
(Unit Operations of Chemical Engineering, McCabe, Smith, Harriot, 5th Edition, McGrawHill)
In practice, distillation may be carried out by either two principal methods:
1. The first method is based on the production of a vapour by boiling the liquid mixture to be separated and condensing the vapours without allowing any liquid to return to the still .There is then no reflux:
- Flash distillation:
- Flash distillation consists of vaporizing a definite fraction of the liquid in such a way that the evolved vapour is in equilibrium with the residual liquid, separating the vapour from the liquid and condensing the vapour.
- Batch distillation:
- In some plants, volatile products are recovered from liquid solution by batch distillation. The mixture is charged to a still or reboiler and heat is supplied through a coil or through the wall of the vessel to bring the liquid to the boiling point and then vaporize part of the batch. In the simplest method of operation, the vapours are taken directly from the still to a condenser.
2. The second method is based on the return of part of the condensate to the still under such conditions that this returning liquid is brought into intimate contact with the vapours on their way to the condenser. Either of these methods may be conducted as a continuous process or as a batch process:
- Continuous distillation with reflux (rectification):
- Flash distillation is used most for separating components that boil at widely different temperatures. It is not effective in separating components of comparable volatility, since then both the condensed vapour and residual liquid are far from pure. By many successive redistillations, small amounts of some nearly pure components may finally be obtained, but this method is too inefficient for industrial distillations when nearly pure components are wanted. Methods now used in both laboratory and plant apply the principle of rectification. In a distillation column, the vapour produced is condensed in a condenser and the liquid is vaporized in a reboiler. Part of these streams is recycled in the distillation column. The recycling liquid stream form the condenser is called reflux. By using plates inside the distillation column, the gas and liquid mixtures are brought into intimate contact and their concentrations tend to move toward an equilibrium state. Some of the more volatile component is vaporized from the liquid, decreasing its liquid concentration, while some of the less volatile component is condensed from the vapour, increasing its vapour concentration. If no azeotropes are encountered, both overhead and bottom products may be obtained in any desired purity if enough plates and adequate reflux are provided.
4. COMPETITIVE TECHNOLOGIES AND ENERGY SAVING POTENTIALS
- a) Changes in the process (BAT on Organic Fine Chemicals, Aug. 2006)
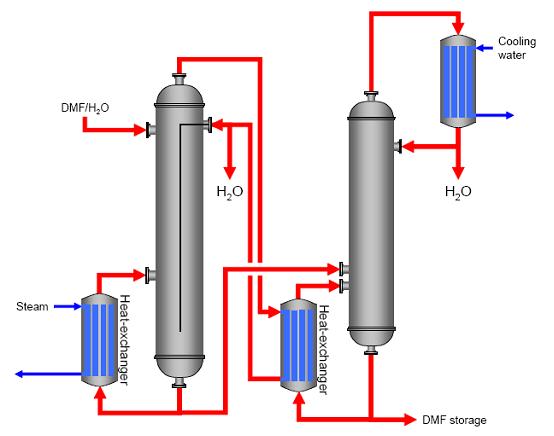
DescriptionIf distillation is carried out in two steps (two columns), energy flows in both columns can be coupled. In this example (purification of DMF, see Figure 1), the steam from the top of the first column is fed to a heat-exchanger at the base of the second column. Steam usage was reduced by about 50 %. This reduction led to cost savings. As a disadvantage, the energy coupling between the two columns means that variations in the first column affect the process in the second column, which can only be handled by improved process control
Figure 1: Energetically coupled distillation of DMF, BREF on Organic Fine Chemicals, Aug. 2006
Achieved environmental benefits
Steam consumption reduced by about 50 %.
Cross-media effects
None believed likely.
Operational data
Depends on the individual case.
Applicability
Generally applicable.
Economics
Cost benefits.
Driving force for implementation
Cost benefits.
- b) Changes in the energy distribution system
No information is available.
- c) Changes in the heat supply system
No information is available.