Organic dyes & pigments
Back to EFFICIENCY FINDER FOR CHEMICAL INDUSTRY
Back to Information about organic pigments and dyes
1 Overview
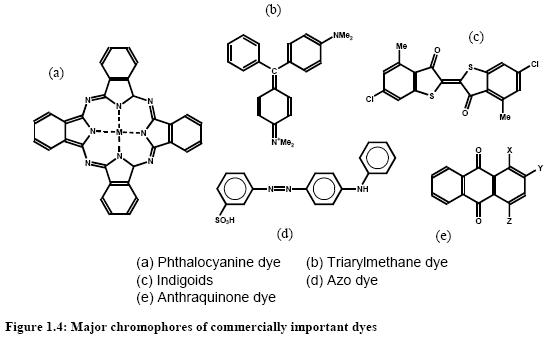
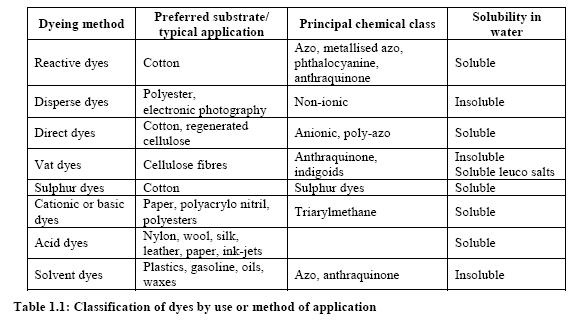
Dyes and pigments can be classified according to their chemical structure or their mode of application. The most important commercial products are the azo, anthraquinone, sulphur, indigoid, triphenylmethane and phthalocyanine dyes. The following figure shows the major chromophores and Table 1 shows the classification of dyes by use or method of application.
Apart from one or two notable exceptions, all dye types used today were discovered in the 1880s. The introduction of the synthetic fibres such as nylon, polyester and polyacrylonitrile during the period 1930 – 1950, produced the next significant challenge. The discovery of reactive dyes in 1954 and their commercial launch heralded a major breakthrough in the dyeing of cotton. Intensive research into reactive dyes followed over the next two decades and is still continuing today. One important theme in research today is the replacement of tinctorially weak chromogens, such as anthraquinone, with tinctorially stronger chromogens, such as (heterocyclic) azo dyes. Considerable activity is also being dedicated to high tech applications, especially in the electronics and non-impact printing industries.
- Pigments
Pigments are defined as colouring agents that are practically insoluble in the application medium, whereas dyes are colouring agents that are soluble in the application medium. In colouring, the crystalline pigment is applied in the solid state, not in the dissolved form, to the medium being coloured. Both the chemical and the physical properties of the pigments (e.g. particulate size, particulate size distribution, special types of surface and specific surface area, crystal modification, and crystal form) are important for their industrial application. Many organic pigments and dyes have the same basic chemical structure. The insolubility required in pigments can be obtained by excluding solubilising groups, by forming insoluble salts (lake formation) of carboxylic or sulphonic acids, by metal complex formation in compounds without solubilising groups, and particularly by incorporating groups that reduce solubility (e.g. amide groups).
The remaining organic pigments (“Other”) are used in textile printing and a number of smaller sectors, including contactless printing processes, office articles and accessories (e.g. coloured pencils, crayons, chalks), and the colouring of wood, cosmetics, and paper.
2 Production of organic dyes and pigments
- Halogenation
Halogenation is one of the most important and versatile processes in chemistry. The industrial application is dominated by chlorinations, due to the different reactivity and the higher price for bromine, iodine and fluorine.
Side chain chlorinated alkyl aromatics, particularly those based on toluene and xylene, as well as nucleus halogenated aromatics, have an exceptional place in organic fine chemistry, because of their role as chemical intermediates in the manufacture of chemical products of almost all kinds, including dyes, plastics, pharmaceuticals, flavours and fragrances, pesticides, catalysts and inhibitors.
Bromination is a key process in anthraquinone chemistry and the manufacture of organic flameretardants.
- Heavily halogenated aromatic hydrocarbons
- Especially as a result of the environmental persistence of the heavily chlorinated benzenes, toluenes and biphenyls, in recent years drastic measures have been applied to this range of chemicals, such as prohibitions, and restrictions on their production and use, and legislation regulating waste disposal. Possible side reactions of the chlorination process can result in the formation of polychlorinated biphenyls or hexachlorobenzene. The combustion of aromatics containing chlorine can lead to the formation of polychlorodibenzo dioxins/-furans (PCDD/PCDF).
- Chemical reaction
- These chemicals are of major relevance on an industrial scale in substitutions of the aromatic nucleus and in the substitution of aliphates. In both cases, hydrogen is replaced by halogen (X) and the related hydrogen halide is created:
- R – H + X2 R – X + HX Ar – H + X2 Ar – X + HX
- Both reactions are exothermic but the aliphate substitution follows a radical chain mechanism, initialised by ultraviolet light (irradiation with mercury vapour lamps), while the halogenation of the aromatic nucleus is based on an electrophilic mechanism supported by Friedel-Crafts catalysts (i.e. Lewis acids such as FeCl3, AlCl3 …).
- Generally, a mixture of isomers and/or compounds with a different degree of halogenation is obtained and side reactions following alternative mechanisms cannot be completely suppressed. The product mix depends on the aromatic/halogen ratio, the reaction conditions and the choice of the catalyst.
- A wide range of organic and aqueous solvents are currently in use, and especially tetrachloromethane, tetrachloroethane, dichlorobenzene and trichlorobenzene are recommended for halogenations.
- Bromine is more efficiently used in aromatic substitution reactions if it is generated in situ from hydrogen bromide using chlorine: ArH + HBr + Cl2 ArBr + 2 HCl
- Another approach is the use of an alcohol as the solvent to co-produce an economically useful alkyl bromide, by the reaction of by-product HBr with the alcohol. Methanol is the solvent of choice since the resulting methyl bromide can be widely marketed as a fumigant.
- Operations
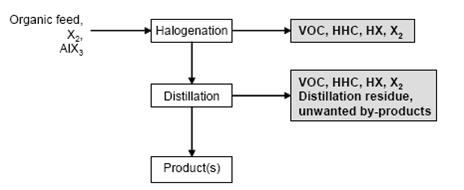
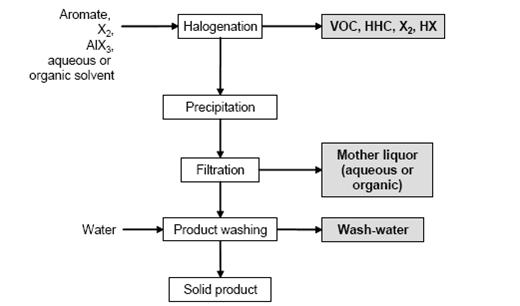
- Figure 1 shows a typical sequence of operations for the halogenation to distillable products. Figure 2 shows a typical sequence of operations for the halogenation precipitation of the product.
Figure 1: Typical sequence of operations for the halogenation to distillable products Possible input materials (on the left) and the associated waste streams (grey background), BREF on Organic Fine Chemicals, Aug. 2006
Figure 2: Typical sequence of operations for halogenation with precipitation of the products Possible input materials (on the left) and the associated waste streams (grey background), BREF on Organic Fine Chemicals, Aug. 2006
- In a typical batch reaction, the halogen is added to the stirred aromatic or a stirred aromatic solution. The reactor material depends on the reactants and the chosen reaction mechanism. The exothermic reaction is controlled by the rate of halogen addition, which is dependent on the refrigeration capacity of the reactor cooling system. The choice of temperature profile is based on the reactivity of the aromatic. On completion of the reaction, degassing is carried out with nitrogen. The product is distilled or precipitated (e.g. by cooling or water addition) and the resulting slurry is filtered, washed and dried. Most side chain chlorinations are carried out continuously or discontinuously in bubble column reactors of enamel or glass, e.g. of the loop type. The reactor is filled with the starting material, heated to at least 80 °C and chlorine is introduced until the desired degree of chlorination is reached. The reaction is stopped by the introduction of N2. Products of different degrees of chlorination are separated by distillation to be directly marketed, hydrolised to give the related benzaldehydes or benzoic acids/benzoyl chlorides, or are used for further chlorination.
- N-acylation
N-acylation is a widely spread reaction for the protection of anilinic amino groups before chlorinations, nitrations or sulphonations are carried out. Arylides (amides of acetoacetic acid) are important intermediates, e.g. for organic pigments.
- Chemical reaction
- The most important N-acylation agents are:
- acetic acid
- acetic anhydride, other carboxylic anhydrides
- diketene
- acetoacetic ester
- acetic chloride, other acyl halides
- N-carboxy anhydrides.
- They work according to the substitution: R’ – NH2 + X – CO – R R’ – NH – CO – R + HX where HX is released. HX may be, e.g. H2O, CH3COOH, C2H5OH, HCl. (the reaction with diketene is an addition).
- Operations
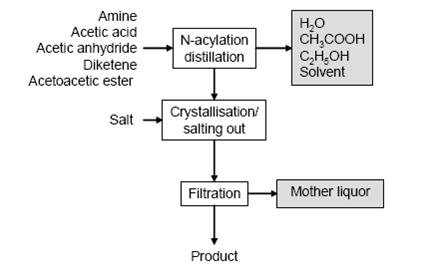
- Figure 3 shows a typical sequence of operations for N-acylations and the typical waste streams. Amine and an equimolar amount of an acylation agent are typically dissolved in H2O or diluted acetic acid (for acetoacetic ester, xylene is often used) and heated. Reaction water or acetic acid or ethanol and solvent are distilled off and the product is obtained directly or following crystallisation (occasionally by salting out) and filtration.
Figure 3: Typical sequence of operations and related waste streams from N-acetylations, BREF on Organic Fine Chemicals, Aug. 2006
- Nitration
Liquid phase nitration is a dominant step in the manufacture of common high explosives and important for the production of a wide range of aromatic intermediates for dyes, agrochemicals, pharmaceuticals or other fine chemicals. A typical nitration reaction is higly exothermic, therefore, for a safe mode of reaction, a dosage controlled process with precautions securing no accumulation of reactants is necessary. Typical nitroaromatic production is based on high yield processes, with more than 80 % of the total cost being the cost of the raw materials. Integral requirements of all efficient nitration processes are sulphuric acid regeneration and isomer control and separation. Nitration of the important naphthalene mono- and disulphonic acids is usually performed with the formed sulphonated mass. Among the typical raw materials are halogenated aromatics, which can contribute to the AOX load of waste water streams.
- Chemical reaction
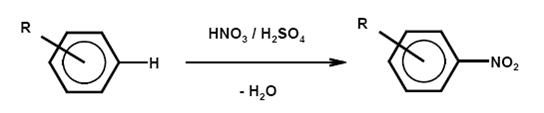
Nitration is the irreversible introduction of one or more nitro groups into an aromatic system by electrophilic substitution of a hydrogen atom. O-nitration to give nitrates and N-nitration to give nitramines are far less important for aromatic compounds but relevant for the manufacture of explosives.
- Nitration is normally carried out in a liquid phase reaction with a mixture of nitric and sulphuric acids (mixed acid) and occasionally with nitric acid. A typical mixed acid, for example for mononitration, consists of 20 % nitric acid, 60 % sulphuric acid and 20 % water (this is referred to as 20/60/20 mixed acid). The strength of the mixed acid and the temperature can be varied to maximise the formation of the required isomer. Stronger mixed acid and higher temperature lead to oxidative side reactions. An important side reaction leads to phenolic by-products.
- Operations
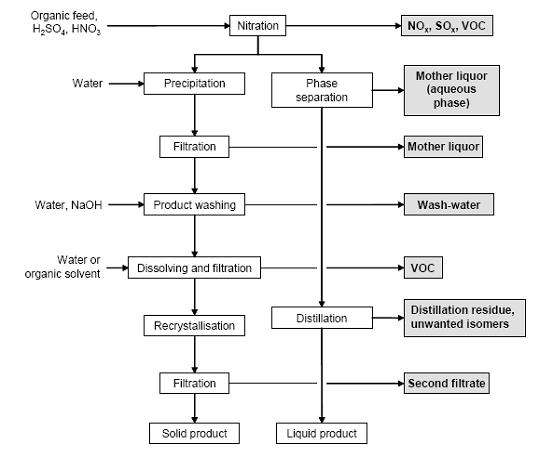
- Figure 4 shows a typical sequence of operations for the nitration of aromatic compounds, possible input materials and associated waste streams. The reaction is carried out in cast iron, stainless steel or enamel-lined mild steel reactors. Temperatures vary normally between 25 and 100 °C. The substrate is dissolved in the sulphuric acid phase and the mixed acid is subsequently added. On completion of the reaction, the batch is transferred into water to give a two phase mixture of diluted acid and an organic product phase. After phase separation, liquid products are purified by distillation. The remaining acid phase can be extracted with the feed material in order to recover organic compounds. :Solid products are crystallised (where necessary, by the addition of cold water). The crude nitroaromatic is washed with water and diluted NaOH to remove the acids and phenolic by-products. Depending on the quality requirements, a recrystallisation from water or organic solvent may be necessary. Isomer separation is carried out within the crystallisation, washing or distillation steps.
Figure 4: Typical sequence of operations for a nitration. Possible input materials (on the left) and the associated waste streams (grey background), BREF on Organic Fine Chemicals, Aug. 2006
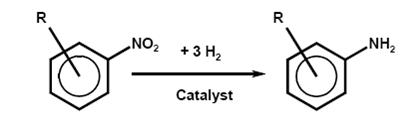
- Reduction of aromatic nitro compounds
One of the most industrially important reduction processes in industrial use is the conversion of an aromatic nitro or dinitro compound into an arylamine or arylene diamine. Aromatic amines are widely used as dye intermediates, especially for azo dyes, pigments, and optical brighteners; as intermediates for photographic chemicals, pharmaceuticals, and agricultural chemicals; in polymers via isocyanates for polyurethanes; and as antioxidants.
Among reduction methods, there are three of major relevance in organic fine chemistry:
- catalytic hydrogenation, which is extremely important industrially because of its universal applicability; most processes can be carried out successfully by catalytic hydrogenation
- Béchamp and Brinmeyr reduction with iron, which is the classical method
- alkali sulphide reduction, which is the selective method in specific cases, such as in the manufacture of nitroamines from dinitro compounds, the reduction of nitrophenols, the reduction of nitroanthraquinones and the manufacture of aminoazo compounds from the corresponding nitroazo derivative.
All three methods are also applied to halogenated nitro compounds, and can thus contribute to AOX loads in waste water streams.
a) Catalytic reduction with hydrogen
- Chemical reaction
- The catalytic reduction of the nitro compounds is very exothermic. To reduce these hazards, the concentration of nitro compound, the amount and partial pressure of the hydrogen, the temperature, and the activity of the catalyst, are controlled.
- Most aromatic nitro compounds are hydrogenated in the liquid phase. In this case, the pressure and temperature can be changed independently. The temperature is limited by the hydrogenation reaction of the aromatic ring which occurs above 170–200°C.
- Normally, the reduction is carried out at 100–170°C. Sensitive compounds are hydrogenated at lower temperatures (20–70°C) or at lower pressures (1–50 bar). 1–50 bar are used normally.
- The preferred solvents are methanol and 2-propanol; and also dioxane, tetrahydrofuran, and N-methylpyrrolidone are used. In the hydrogenation with a water immiscible solvent, such as toluene, the water must be removed, as in solvent-free hydrogenation, in order to maintain the activity of the catalyst. If the amine has a good water solubility, water is used as the solvent. Water also can be used in cases where the nitro compound forms water-soluble salts with alkalis, such as with nitrocarbonic or sulphonic acids. In practice, only Raney nickel, Raney nickel-iron, Raney cobalt, and Raney copper are used as pure metal catalysts because of their relatively low cost. Precious metal catalysts, such as Pt and Pd, are generally used at concentrations of 0.5 – 5 wt-% on support material with large surfaces, such as charcoal, silica, aluminium oxide, or alkaline-earth carbonates.
- Process hazards
- The catalytic reduction of nitro compounds is very exothermic. Unless this heat is dissipated properly, decomposition and even explosions can result, especially if the thermal decomposition of the nitro compound occurs or if condensation reactions are initiated as may be the case with chloro-nitro compounds. The industrial hydrogenation of aromatic polynitro compounds in the liquid phase without solvents especially requires precautions. To reduce these hazards, the concentration of the nitro compound, the amount and partial pressure of the hydrogen, the temperature, and the activity of the catalyst are controlled. The nitro compound is continuously added in small quantities, thus keeping its concentration below 2%. De-ionised water is added to remove the heat of the reaction by continuous evaporation and to slow down the activity of the catalyst.
- Operations
- The vast majority of aromatic amines have small annual volumes (<500 tonnes) and are produced by batch hydrogenation with catalyst slurries. The reaction is carried out in stirred, steel or stainless steel autoclaves or in loop reactors. Loop reactors show increased heat and mass transfers and improved reaction selectivity, shorter batch cycle times and higher product yields. In addition, catalyst usage is often lower. The addition sequence depends on the particular reactants. On completion the reaction mass is cooled and the catalyst is removed by filtration.
b) Reduction with iron
- Chemical reaction
- The reduction of nitroaromatics is carried out in the presence of small amounts of acid (HCl, H2SO4, HCOOH, CH3COOH) as shown in the following equation:
- 4 Ar – NO2 + 9 Fe + 4 H2O 4 Ar – NH2 + 3 Fe3O4
- The acid is used for the activation of the iron. Only 2 – 3 % of the hydrogen is derived from the acid but 97 – 98 % comes from the water.
- Operations
- Normally the nitroaromatic is added to the mixture of iron/water/acid (excess of iron about 15-50 %) often in the presence of an organic solvent (toluene, xylol, alcohols) and the mixture is heated to reflux. Depending on the reactivity of the aromatic, other addition sequences may be required. In some cases the acid is omitted (neutral iron reduction). The build-up of unreduced excess nitro compound must be avoided and the final mixture should be tested for its total absence. After basification with soda ash (anhydrous sodium carbonate) to precipitate soluble iron, the iron compounds are removed by filtration.
c) Alkali sulphide reduction
- Chemical reaction
- The alkali sulphide reduction is a mild and selective reaction according to the following equation, without strict stoichiometry:
- Ar – NO2 + Na2S2 + H2O Ar – NH2 + Na2S2O3
- Other reducing agents in use are Na2S or NaSH, which also form Na2S2O3. Sulphur may be added to reduce the required amount of sulphide.
- Operations
- Dilute aqueous sulphide is added to the solution or emulsion of the nitro compound. Temperatures (in the range of 80–100°C) and concentrations depend on the reactivity of the nitroaromatic. An excess of sulphide is avoided in the case of the selective reduction of polynitro compounds.
PRODUCT WORK-UP:
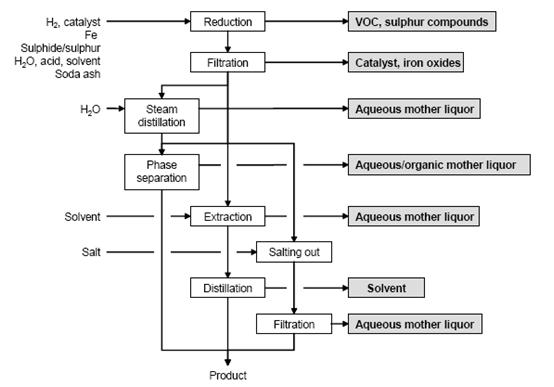
Figure 5 shows a typical sequence of operations for the reduction of aromatic nitro compounds, possible input materials and associated waste streams. The work-up depends on the properties of the amine obtained. Common methods are:
- separation as a liquid
- cooling and salting out
- steam distillation
- extraction with organic solvent, and
pH adjustment if necessary.
Figure 5: Typical sequence of operations for the reduction of an aromatic nitro compound. Possible input materials (on the left) and the associated waste streams (grey background), BREF on Organic Fine Chemicals, Aug. 2006
3 Economics
The scale and growth of the dyes industry is linked to that of the textile industry. World textile production has grown steadily to an estimated 35 million tonnes in 1990. The two most important textile fibres are cotton and polyester. Consequently, dye manufacturers tend to concentrate their efforts on producing dyes for these two fibres. The estimated world production of dyes and pigments in 1990 was 1 million tonnes. The rapid growth in the high tech uses of dyes, particularly in ink-jet printing, is beginning to make an impact. Although the volumes in this area remain small in comparison to dyes for traditional applications, the value will be significant because of the much higher price.
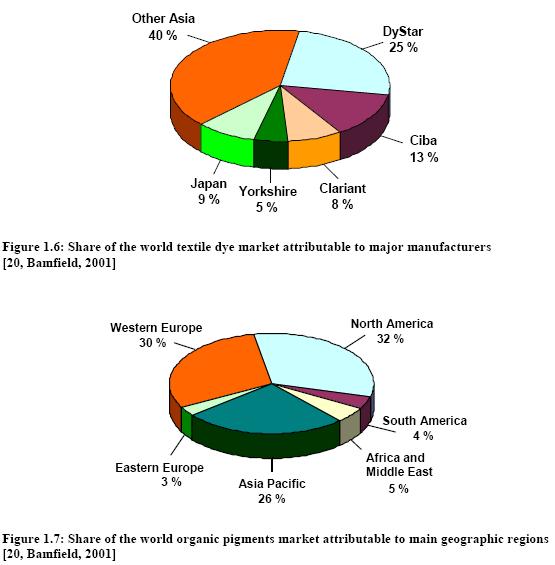
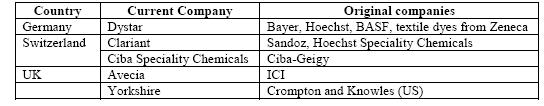
The Western European share of world production has declined from 95 % in the early 1900s to about 40 %, taking into account that a large part of US manufacture and that of other countries is based on Western European subsidiaries. This decline is coupled with an increase of production in commodity dyestuffs in lower cost countries such as India, Taiwan and China. The world output of organic dyes is estimated to be 750000 tonnes per year [6, Ullmann, 2001]. The major European dye manufacturers have undergone major reorganisations, mergers and acquisitions to focus on “core” activities (Table 1.2).
Literature: BAT for Manufacture of organic fine chemicals, August 2006