Polypropylene (PP)
Back to Information about polyolefins
1 General description
Polypropylene (PP) is one of the economically most important thermoplastic materials.
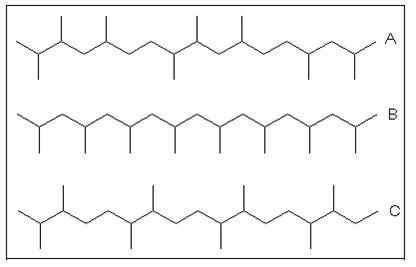
PP is a linear polymer and is classified as a polyolefin. The methyl (CH3) group is characteristic. Depending on the spatial arrangement of these groups to the main -CC-chain, one differentiates between atactic PP (aPP) with an irregular CH3 arrangement, isotactic PP (iPP) with CH3 groups on one side of the carbon chain and syndiotactic PP (sPP) with an alternating CH3 arrangement as shown in the following Figure. Increasing the tacticity (regularity of the CH3 arrangement) leads to an increase in the degree of crystallinity, fluxing temperature, tensile strength, rigidity and hardness.
- A) atactic polypropylene
- B) isotactic polypropylene
- C) syndiotactic polypropylene
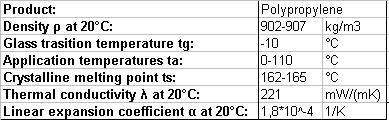
Table 1: Properties of PP, Literature: Hirschberg, H. G.: Handbuch Verfahrenstechnik und Anlagenbau
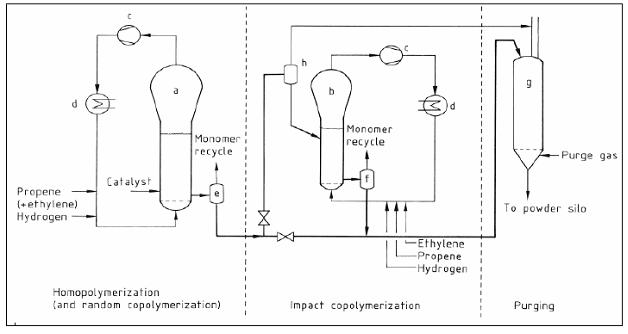
2 Flow diagram of HDPE production
- Suspension processes
Figure 2: Generic flow diagram showing the traditional suspension (‘slurry’) process
- Suspension processes : Shperipol process
- A) loop reactors
- B) primary cyclone
- C) copolymer fluidised bed
- D) secondary and copolymer cyclone
- E) deactivation
- F) purging
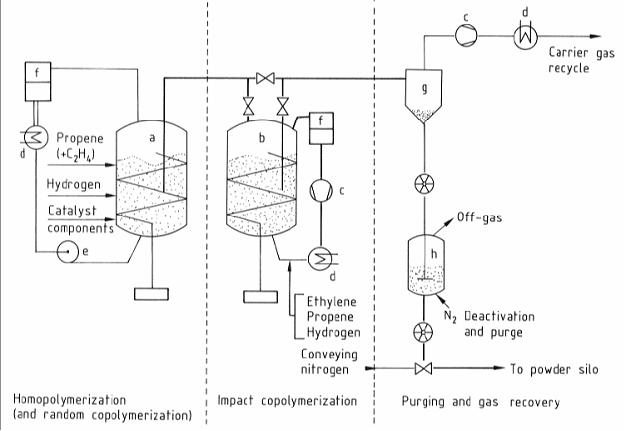
- Gas phase processes : fluidized bed reactor
Figure 4: Flow diagram of the polypropylene fluidised bed gas phase process
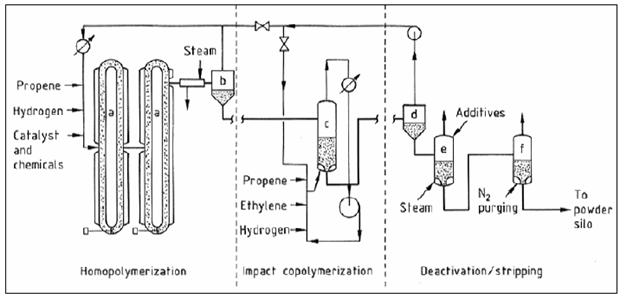
- Gas phase processes : vertical reactor
- A) primary reactor
- B) copolymeriser
- C) compressors
- D) condensers
- E) liquid pump
- F) filters
- G) primary cyclone
- H) deactivation/purge
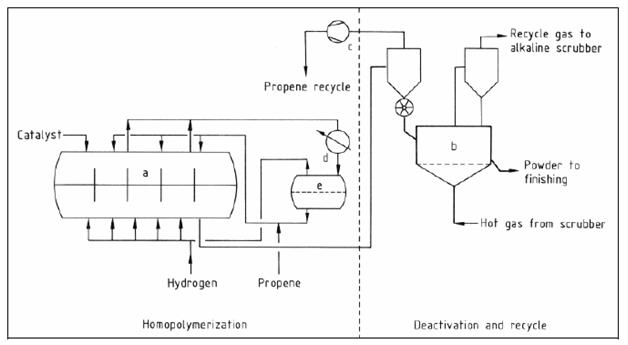
- Gas phase processes: horizontal reactor
Figure 6: Flow diagram of the polypropylene horizontal reactor gas phase process
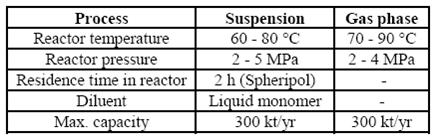
3 Technical parameters
4 Competitive technologies and energy saving potentials for the production of PE
- Solvent and comonomer selection for PE processes
Description
A solvent is needed as the carrier for catalyst or initiator feeds or as the reactor diluent for the solution and slurry suspension processes, while a comonomer is used to control the polymer density of the final product. In principle, the more volatile the hydrocarbon solvent and comonomer, the easier the separation from the polymer will be. However, there are some practical limitations:
- comonomer selection – comonomer selection is dictated by product design, desired application properties and targeted product value
- solvent selection for the LLDPE solution process – typically, the solution process is used to make higher value LLDPE grades through the application of hexene-1 or octene-1 as the comonomer. These comonomers are compatible with the hydrocarbon solvents, typically in the range of C6 to C9, used in the reactor system. Lower boiling comonomers and/or solvents are, in principle, possible, but might require a higher reactor operating pressure and thus more energy, to avoid phase separation and to maintain single phase conditions
- solvent and comonomer selection for the LLDPE gas phase process – the use of butene-1 as the comonomer results in very low residual hydrocarbon levels in the polymer fed to the extrusion section. However the use of hexene-1 as the comonomer (to improve product value) and/or the use of a condensable solvent (to improve plant productivity and energy consumption) will increase the residual hydrocarbon content
- solvent selection for the slurry HDPE suspension process – in principle, the more volatile the suspension solvent is the easier the removal, however low boiling solvents require a more complex condensing/recovery system. Furthermore, plant design (unit operations and design pressure) can prevent the application of low boiling solvents in the range of C4 to C6
- solvent selection for the high pressure polyethylene process – solvents are used as a carrier for the initiator in order to facilitate stable injection of the applied initiator. In principle, two types of solvents can be used in the high pressure polyethylene process, namely lower boiling hydrocarbon solvents in the range of C7 to C9 and higher boiling solvents in the range of C10 to C12. A lower boiling solvent will facilitate removal from the product, but leads to higher levels building up in the ethylene recycling systems. A higher boiling solvent is more difficult to remove from the polymer, but is easier to condense in the recycling streams and will thus lead to lower levels in the recycling systems. The net effect on the residual solvent level might be neutral. Good practice in the high pressure polyethylene process is to minimise the usage of hydrocarbon solvents while maintaining a stable injection of the initiators to the reactor system. Larger scale operations help to reduce the unit solvent consumption and also the residual solvent level in polymer.
Achieved environmental benefits
Low boiling solvents and suspension agents can be removed more easily and with less consumption of energy from the product leading to a reduction of VOC emissions from the storage.
Cross-media effects
No further information submitted.
Operational data
No further information submitted.
Applicability
As described. For some speciality products, such as PP for capacitor films, less volatile diluents are used to guarantee the product quality.
Economics
No further information submitted.
Driving force for implementation
No further information submitted.
- Increase of the polymer concentration in the reactor system to the maximum possible
Description
By increasing the concentration of the polymer in the reactor, the overall energy efficiency of the production process is optimised as follows:
- high pressure polyethylene process – in the high pressure polyethylene process this is achieved by maximising heat transfer. However, the properties of the product and the concentration of the polymer in the reactor are interdependent. The desired product quality imposes, therefore, a limitation to the maximum ethylene conversion level possible
- solution processes – the maximum polymer concentration in the solution process is a function of maximum temperature capability of the catalyst system, heat removal capability and maximum allowable process viscosity
- gas phase processes – in principle, there is no limitation as long as fluidised conditions and homogeneous temperature conditions are maintained in the reactor system. The recycling energy is reduced by improving heat removal by the addition of a condensable solvent and/or comonomer in the reactor system (so called ‘condensation mode’)
- HDPE slurry suspension process – the maximum viscosity of the slurry limits the maximum concentration of polymer solids in the hydrocarbon diluent. The slurry has to be maintained transportable. Depending on particle size distribution this means that typically the solid concentration has to be maintained between 30 and 35 vol-%.
Achieved environmental benefits
Increase of energy efficiency.
Cross-media effects
No further information submitted.
Operational data
No further information submitted.
Applicability
No further information submitted.
Economics
No further information submitted.
Driving force for implementation
Economic and environmental reasons.
Literature: BAT for Polymers, October 2006
Back to Information about polyolefins