Vitamins
Back to EFFICIENCY FINDER FOR CHEMICAL INDUSTRY
Back to Information about pharmaceutical ingredients and vitamins
1 Overview
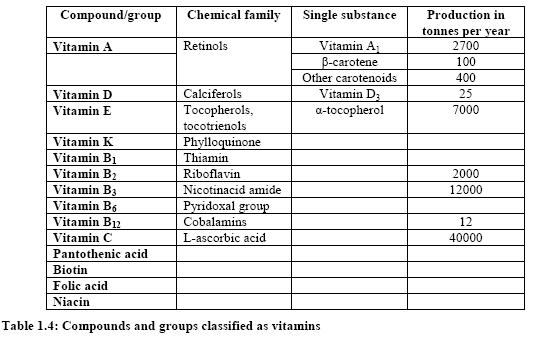
Vitamins are essential, organic compounds which are either not synthesised in the human and animal organism or which are formed only in insufficient amounts. Pro-vitamins can be converted to the vitamin in the body. A typical representative of the pro-vitamins is U-carotene, which is split into two molecules of vitamin A in the organism. Vitamins are classified not chemically but by their activity. The historical distinction between fat- and water-soluble vitamins has been retained to this day, since the solution properties are important not only for the occurrence, but also for the behaviour of vitamins in the organism (resorption, transport, excretory pathways, and storage). Fourteen compounds or groups of compounds have been classified as vitamins (Table 1.4). The worldwide market value for vitamins as a bulk product is estimated to be EUR 25600 million (DEM 50000 million) per year [6, Ullmann, 2001].
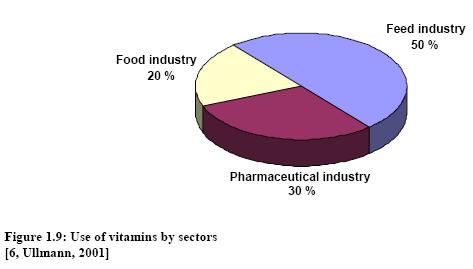
2 Production of vitamines
- Esterification
Organic esters are of considerable economic importance. Because of their highly lipophilic and hydrophobic nature and low polarity, esters are widely used as solvents, extractants, and diluents. Ethyl acetate is the most common technical solvent. Large quantities of esters, especially phthalates, adipates, and fatty acid esters, are used as plasticisers. Esters with a pleasant odour are used in fragrances, flavours, cosmetics, and soaps. Esters can be converted into various derivatives and are useful intermediates in the synthesis, e.g. of vitamins or pharmaceuticals.
Chemical reaction
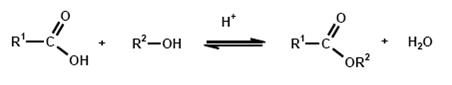
A great variety of production methods for carboxylic acid esters are known, but the simplest and most common method of esterification is the reaction of an alcohol with a carboxylic acid with the elimination of water:
Esterification is the reverse of hydrolysis and leads to an equilibrium reaction, which is the reason that quantitative esterification is possible only by continuous removal of one of the products, i.e. ester or water. In the case of transesterification, an alcohol is released instead of water.
Suitable catalysts are sulphuric acid, hydrogen chloride, arylsulphonic acids such as p-toluenesulphonic acid, and chlorosulphuric acid. Phosphoric acid, polyphosphoric acids, and mixtures of acids are also recommended. If the acids are adsorbed on a solid support, esterification can be carried out as a continuous process.
Removal of water usually involves the addition of entrainers, which form azeotropes with relatively low boiling points and high water contents (usually toluene, xylene, cyclohexane, seldom also benzene or CCl4).
Operations
The reaction is generally carried out by refluxing the reaction mixture until all the water has been split off. The water or the ester is removed from the equilibrium by distillation. Water is usually removed by distillation of the azeotrope with the alcohol or an entrainer. After condensation, the azeotrope separates into an aqueous phase and an organic phase, and the entrainer or alcohol is recycled into the reaction mixture. In particular cases, a co-solvent such as benzene or toluene is added to the condensate to achieve separation of the organic phase. Many esters are produced continuously in pipes, distillation columns or plate columns. Ionexchange resins are especially suitable as catalysts in continuous processes. The reactants pass through or over the solid catalyst, and no separation or neutralisation of the catalyst is necessary.
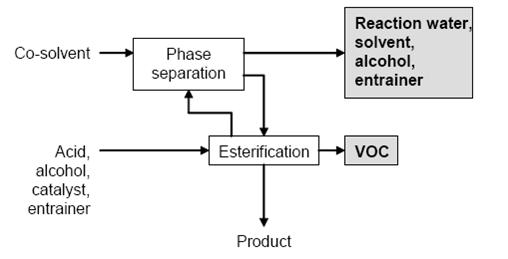
Figure 1: Typical sequence of operations for esterification Possible input materials (on the left) and the associated waste streams (grey background)
Literature: BAT for the Manufacture of organic fine chemicals, August 2006
Back to EFFICIENCY FINDER FOR CHEMICAL INDUSTRY
Back to Information about pharmaceutical ingredients and vitamins