Plasticisers
Back to Information about plasticisers
1 Overview
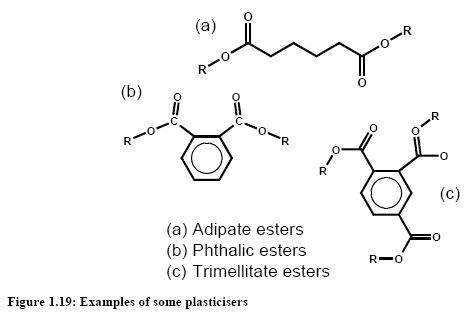
A plasticiser is a substance incorporated into a material to increase its flexibility, workability, or distensibility. A plasticiser may reduce the melt viscosity, lower the temperature of the secondorder transition, or lower the elastic modulus of the product. Plasticisers are inert, organic substances with low vapour pressures, predominantly esters, and react physically with high polymers to form a homogeneous physical unit, whether it be by means of swelling or dissolving or any other. At present some 300 plasticisers are manufactured, of which at least 100 are of commercial importance. Figure 1.19 shows some of the most widely used plasticisers.
In 1996, production of plasticisers in Western Europe amounted to 1253 × 103 tonnes per year and in the United States to 636 × 103 tonnes per year [99, D2 comments, 2005]. In terms of plasticiser types, the majority of this tonnage (>85 %) is standard phthalate (esters of phthalic anhydride with C8 – C10 alcohols). The reasons for this are the relatively low price and readily available feedstocks. The remainder of the market is taken up by phthalate esters of other alcohols, speciality phthalates, adipates, trimellitates, and other esters.
2 Production of plasticisers
- Esterification
Organic esters are of considerable economic importance. Because of their highly lipophilic and hydrophobic nature and low polarity, esters are widely used as solvents, extractants, and diluents. Ethyl acetate is the most common technical solvent. Large quantities of esters, especially phthalates, adipates, and fatty acid esters, are used as plasticisers. Esters with a pleasant odour are used in fragrances, flavours, cosmetics, and soaps. Esters can be converted into various derivatives and are useful intermediates in the synthesis, e.g. of vitamins or pharmaceuticals.
Chemical reaction
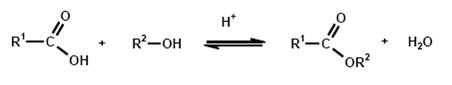
A great variety of production methods for carboxylic acid esters are known, but the simplest and most common method of esterification is the reaction of an alcohol with a carboxylic acid with the elimination of water:
Esterification is the reverse of hydrolysis and leads to an equilibrium reaction, which is the reason that quantitative esterification is possible only by continuous removal of one of the products, i.e. ester or water. In the case of transesterification, an alcohol is released instead of water.
Suitable catalysts are sulphuric acid, hydrogen chloride, arylsulphonic acids such as p-toluenesulphonic acid, and chlorosulphuric acid. Phosphoric acid, polyphosphoric acids, and mixtures of acids are also recommended. If the acids are adsorbed on a solid support, esterification can be carried out as a continuous process.
Removal of water usually involves the addition of entrainers, which form azeotropes with relatively low boiling points and high water contents (usually toluene, xylene, cyclohexane, seldom also benzene or CCl4).
Operations
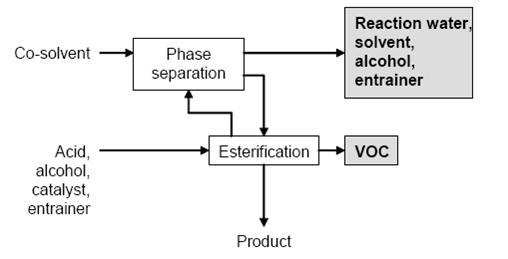
The reaction is generally carried out by refluxing the reaction mixture until all the water has been split off. The water or the ester is removed from the equilibrium by distillation. Water is usually removed by distillation of the azeotrope with the alcohol or an entrainer. After condensation, the azeotrope separates into an aqueous phase and an organic phase, and the entrainer or alcohol is recycled into the reaction mixture. In particular cases, a co-solvent such as benzene or toluene is added to the condensate to achieve separation of the organic phase. Many esters are produced continuously in pipes, distillation columns or plate columns. Ionexchange resins are especially suitable as catalysts in continuous processes. The reactants pass through or over the solid catalyst, and no separation or neutralisation of the catalyst is necessary.
Figure 1: Typical sequence of operations for esterification Possible input materials (on the left) and the associated waste streams (grey background)
Literature: BAT for the manufacture of organic fine chemicals, August 2006
Back to Information about plasticisers