Process description: Immersion coatings
Back to Immersion coatings in metal industry
- Immersion or displacement coatings – non-catalytic chemically reduced coatings
Non-catalytic chemically reduced coatings have been in use for many years, and are often known as immersion or displacement coatings. They are formed when the metal to be deposited is precipitated on its reduction in solution either (i) chemically form solution, or (ii) the metallic substrate is more active than the ions in the solution in terms of the electromotive or electrochemical series, e.g.
Cu2+ + Fe0 -> Cu0 + Fe2+
Although these deposits are often non-adherent and of poor physical quality, careful attention to solution composition and operating conditions can produce deposits that are acceptable for certain purposes. The zincate and stannate solutions used for plating aluminium are examples of special finishes producing acceptable deposits.
- Printed circuit boards
High purity deposits, usually one of gold, silver and tin, are widely used on printed circuit boards. Thickness does not exceed 0.1 – 0.2 μm.
- Mirrors
Their best known use is in producing mirrored surfaces from silver, although other techniques can now be used (such as vapour phase deposition). Their two main drawbacks are that only relatively thin coatings can be deposited and that all surfaces, including the container, receive a coating. While these solutions are sometimes used by immersion, they are now more often applied by spraying the solutions form a dual spray gun. The first step in silver coating of mirrors is the activation of the glass surface by stannous chloride (SnCl2). This is followed by the application of a solution of silver nitrate (AgNO3) and a reducing agent (usually glucose) by spraying. The precipitated silver (about 12 – 17 μm thick is rinsed by deionised water. The Ag coating is fixed by cementing copper with iron or zinc, forming a layer several μm thick: the copper is precipitated form solution by contact with the more electropositive silver deposit. This technique was discovered in 1835 and is still the best one. Some years ago a Belgian patent was given for a technique which used tin for fixation of Ag coating solution of Sn. This process is used by the largest producer of mirrors in the Czech Republic. Passivation using mercaptosilanes is also used.
The metallic layers are protected by a 50-70 μm of solvent-based lacquers. Replacement by water-based lacquers has so far been unsuccessful.
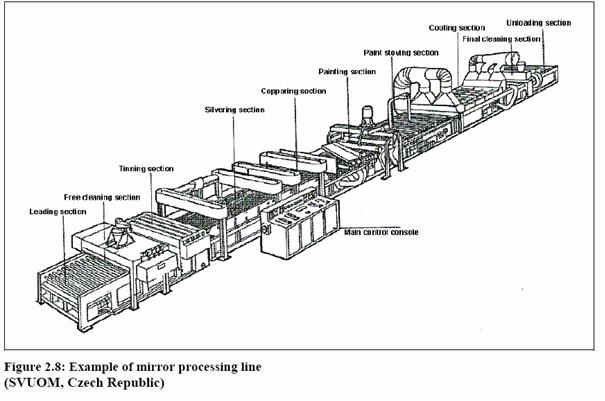
The following shows an example of a mirror processing plant:
Source: BAT Surface Treatment of Metals and Plastic, Aug. 2006.